Tucker Carlson’s interview with siblings Calley and Casey Means on August 16, 2024.
Calley: There’s actually no dynamic in American capitalism like the vaccine schedule because the second you get something on that schedule, the government is paying hundreds of billions of dollars for a product that’s then mandated for every single American living.
Tucker: We’re talking math here.
Calley: Working with the pharma industry, it’s a huge economic imperative to get more and more vaccines on the schedule. … This is big business, right? Hundreds of billions of dollars. And, again, once you get it approved, what happens? It’s paid for for everyone, and you have the most trusted institutions in the world calling anyone a war criminal for even asking a question about it.
As Calley noted, the CDC’s recommended childhood vaccine schedule is big business. Some vaccines on the market will never make it on the schedule, like rabies, shingles, and yellow fever, so they will never be as lucrative as those on the childhood schedule.
Why is the childhood schedule a golden ticket?
The National Childhood Vaccine Injury Act (NCVIA) identifies only those vaccines on the CDC’s recommended childhood vaccine schedule as those receiving a “get out of jail free” card. For an industry where almost all participants have criminal records1 and are well known for products that have caused catastrophic harm, the NCVIA is a siren song. Passed by Congress in 1986, NCVIA removes all liability for only those vaccines on the childhood schedule, thus creating an extremely lucrative business model and a boom in the childhood vaccine market. Of course, with zero product liability, there’s no incentive to determine how or if the vaccines recommended to every American infant and child play a role in creating the chronic disease epidemic plaguing children today. And the federal government doesn’t demand the studies because it is the one pushing the schedule.
“An estimated 43% of US children (32 million) currently have at least 1 of 20 chronic health conditions assessed, increasing to 54.1% when overweight, obesity, or being at risk for developmental delays are included; 19.2% (14.2 million) have conditions resulting in a special health care need. . .”2
While the nation is finally focused on this monumentally important topic, let’s take a closer look at the CDC’s recommended childhood vaccine schedule, a document that not only provides the pharmaceutical industry with billions in profits, but also influences every state’s laws and policies.
Childhood schedule at-a-glance
- The CDC’s first list of recommendations was published in 1969. Prior to the CDC’s schedule, childhood immunization recommendations were handled by the American Academy of Pediatrics.
- The CDC schedule is a policy document. In other words, the schedule is a suggestion or recommendation. While it is not law, over the years, it has been used to substantiate state laws, as well as school and employment policies.
- Insurance payments for immunizations are tied to the schedule updates, not recommendations that shift or grow through the year.
- Once vaccines go on the schedule, they don’t come off. Two vaccines were temporarily removed from the schedule — DTP and RotaShield — but replaced with different versions and put back on. The U.S. childhood immunization schedule is published annually, but as of September 2023, rolling updates will be added to a newly created addendum.
- Also new as of September 2023, the CDC introduced a new “Schedule by Medical Indication” for children, which includes recommendations for pregnancy in children, HIV infection, kidney failure, heart disease, and more.
History of CDC schedules
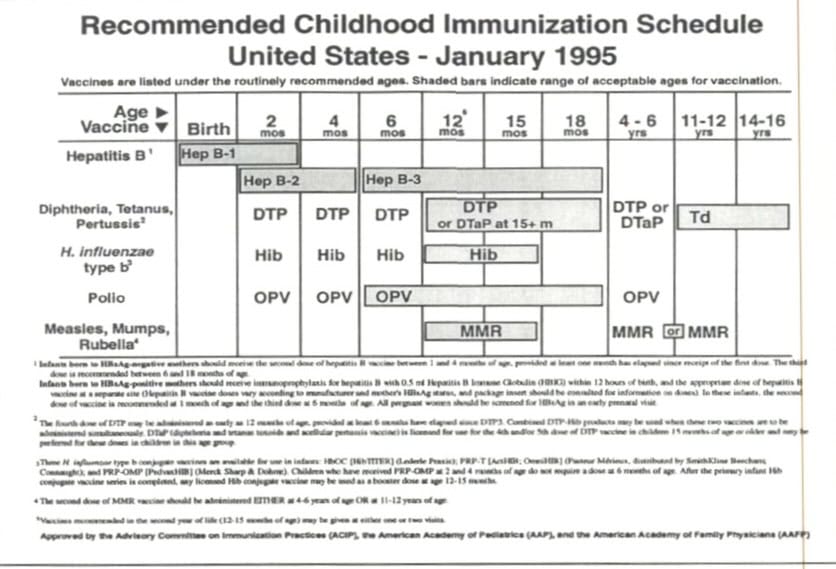
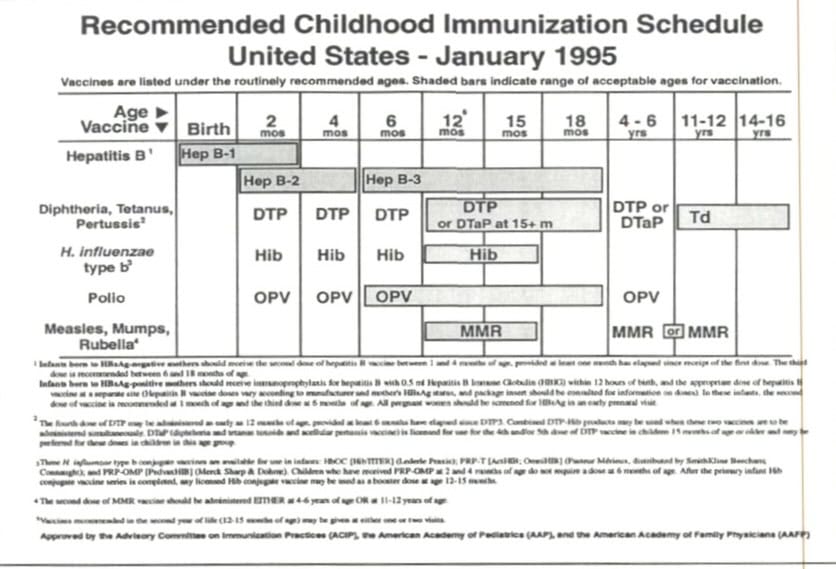
When the CDC became involved in making childhood immunization recommendations in 1969, they suggested shots for eight diseases: diphtheria, tetanus, pertussis, measles, mumps, rubella, poliomyelitis, and smallpox. The recommendations consisted of up to 32 doses in the first 18 years of life, but most children got 24. (The more popular oral polio vaccines were 16 doses and boosters, while the shot was 24). The first shot schedule, in table form as we know it now, was introduced in 1995.
Today the CDC recommends upwards of 80 doses for 18 illnesses. Malaria, tuberculosis, and shigella vaccines are in development3 and there’s already a buzz of GBS being added soon for pregnant mothers. Interestingly, with the addition of a monoclonal antibody therapy for RSV on the adult schedule, the phrase “Vaccines and Other Immunizing Agents” is now incorporated into the adult schedule.
Compare the 1995 “first” schedule to today’s schedule in mid-2024.4
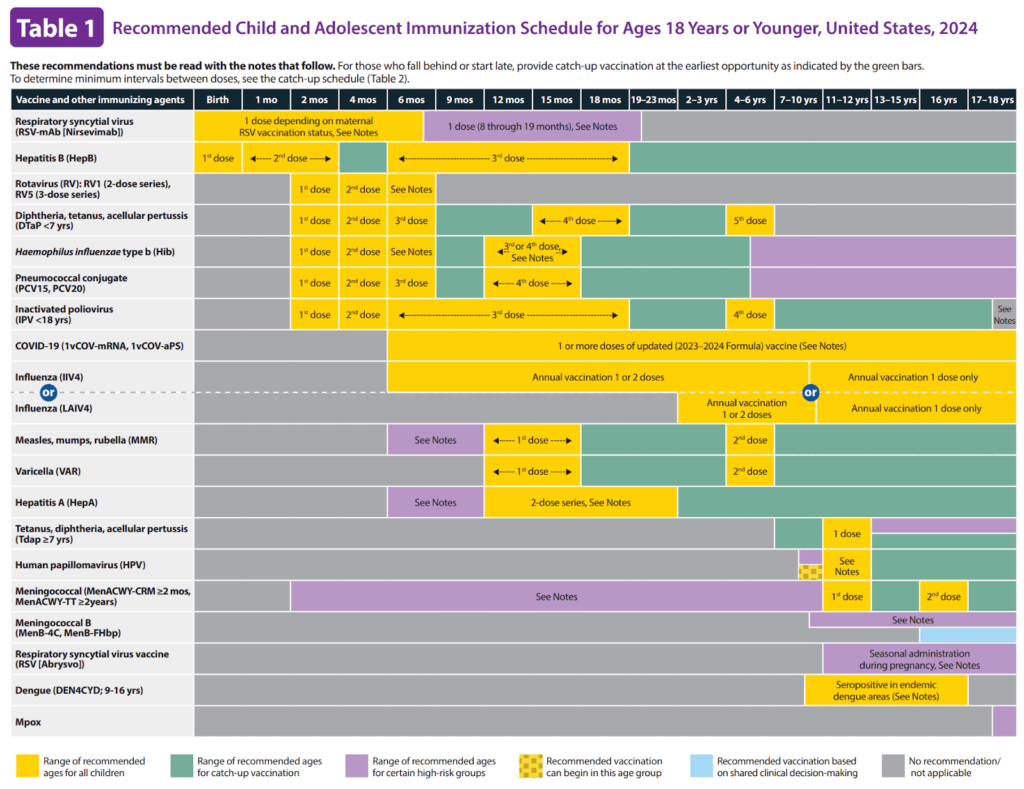
Doctors went from recommending 32 doses for eight illnesses in the 1960s, to upwards of 80 doses for 18 illnesses in 2024. What happened?
How did we get here?
The original Communicable Disease Center was founded in 1946 to “be the servant of the states, providing practical help whenever called,”5 and was staffed by former employees of the Office of Malaria Control in War Areas. Today, after several name changes, the Centers for Disease Control and Prevention (CDC) holds power over a document that opens (or closes) doors to education and employment across the country, ensures billions in profits for vaccine manufacturers when their product is added to it, and determines what preemptive medical interventions insurance companies will pay for. Yet after decades of vaccine recommendations, some as long as a century, and despite claims that vaccines will eliminate disease, only one virus (smallpox) has been declared “eliminated.” Smallpox inoculation was a CDC recommendation through the 1970s, but it was never on the schedule as we know it now, since the graphic table of shots and doses wasn’t introduced until 1995.
Who decides what’s added to the schedule?
The Advisory Committee on Immunization Practices (ACIP) was “chartered as a federal advisory committee to provide expert external advice to CDC”6 and convened their first meeting on May 25, 1964. The 15 voting members are people put forth as experts in preventing communicable diseases, including one member of the public, and meant to advise the CDC on recommendations. The committee was created to centralize recommendations for preventing communicable diseases. Usually, the CDC will align with the ACIP’s recommendations, but on some occasions, the director may override their decisions, as Rochelle Walensky did in 2021 when the ACIP voted not to recommend another COVID booster shot for health care7 workers. Pressured by health care lobbying associations, Walensky approved the COVID booster shot recommendation for health care workers stating, “In a pandemic, even with uncertainty, we must take actions that we anticipate will do the greatest good.” In other words, the CDC director caved to the pressure. Without data to support the decision, she negated the vote of the ACIP’s 15-member panel and recommended the COVID booster for all health care workers.
From the very start, there were discussions of “simplification” of the vaccine schedule. The goal was not to minimize vaccines, but instead to make it more simple for doctors to recommend them and for parents to want their kids to get them. But the schedule has ballooned beyond imagination, even including an addendum providing updates throughout the year. The Affordable Care Act linked payments to the schedule, but did not define the schedule. Therefore, the CDC has decided the schedule is not just the graphic that we are all familiar with, but also any other notes or addendum that accompany that graphic.
When we hear talk of “simplification” now, it’s about how to make bigger combination shots like the new Vaxelis, meant to cover six illnesses in one shot, or how to “bundle” the time kids get shots together because you’re already in the office anyway, such as adding HPV to recommendations for 11-year-olds already expected to get meningococcal and Tdap.
Before the ACIP, the American Academy of Pediatrics (AAP) — the professional organization — had dominion over vaccine recommendations for children, which they published in something called their Red Book. Now, nine professional health-care-related organizations, most funded by pharmaceutical companies, partner with the CDC to approve vaccine schedules, which are explained in the Pink Book.
- American College of Physicians
- American College of Family Physicians
- American College of Obstetricians and Gynecologists
- American College of Nurse-Midwives
- American Academy of Physician Associates
- American Pharmacists Association
- Society for Healthcare Epidemiology of America
- American Academy of Pediatrics
- National Association of Pediatric Nurse Practitioners

“Addition of an Addendum to the 2023 immunization Schedules for Children/Adolescents and Adults. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-22/09-Mat-Peds-Schillie-508.pdf
In 1964, the newly formed ACIP team felt that “14 separate visits in 16 years” was not the best way to push the vaccines. But given the complexity of spacing of doses, they decided instead to first focus on “a definitive review of the DTP problem,” referring to the increasing reports of injuries and death after the combination DTP shot. More than 30 years later and a decade after the National Childhood Vaccine Injury Act was passed, in part, because of the serious harms caused by the DTP vaccine, that vaccine was finally taken off the schedule and off the American market in 1996. Despite the known harms associated with DTP at the time, the next diphtheria, tetanus, pertussis vaccines added to the schedule to replace DTP used the DTP vaccine as the “placebo” in the clinical trials.,8,9 The two DTaP vaccines currently on the market in the U.S. – Infanrix and Daptecel – used DTP as the “placebo” in the clinical trials.10,11 DTP is still used outside of the U.S. despite peer-reviewed data indicating “that DTP vaccine may kill more children from other causes than it saves from diphtheria, tetanus or pertussis.”12
In 1999, three years after DTP was removed from the CDC schedule, at the height of the thimerosal and Wakefield controversies, RotaShield, a rotavirus vaccine, was removed from the schedule. Sacrificed as evidence that the safety process works and to bolster public confidence in the vaccine program, RotaShield was withdrawn to protect public perception of vaccine safety in general.13 Commenting on RotaShield, Dr. Paul Offit, co-inventor of Rotateq rotavirus vaccines, stated:
“We said this [RotaShield] is unsafe for American children, period, without ever defining safety. What we meant by doing it the way we did was absolute safety, which isn’t a reasonable definition. It’s a lawyer’s definition. It’s not a doctor or scientist’s definition.” 14
In other words, even with a significant adverse event profile, it may take years for a vaccine to be removed from the CDC schedule, if at all. Offit’s comments regarding RotaShield give insight into why shots like HPV or COVID-19 have not been taken off the market yet: absolute safety is not required.
Children are at high risk for. . . mandates
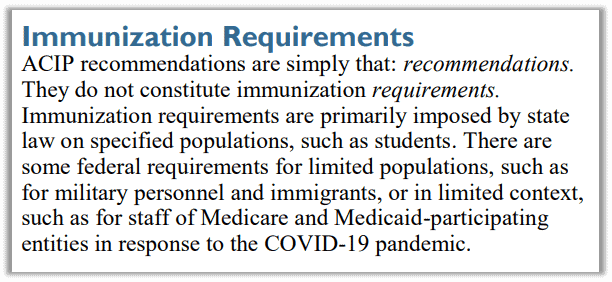
Notably, at the first ACIP meeting it was acknowledged that “the primary responsibility for public health immunization activities rests with the individual States and their State Health Officers.” The CDC cannot do anything without states’ buy in. The CDC can recommend all it wants, but it’s up to individual states to choose which, if any, immunizations to require for schools or other activities.
Despite this clear acknowledgement that ACIP recommendations are just that, recommendations, every state has implemented either policy or legislation to ensure compliance, most often on school-aged children.
In addition to state school mandates, what’s tied to the schedule?
- Insurance payments. Many insurers link payment to the vaccine being on the schedule.
- What a pharmacist can do. Pharmacists typically cannot administer something not on the schedule.
- Doctor’s recommendations. It is standard practice for doctors to refer to the schedule.
The Affordable Care Act states that insurers must provide coverage for and must not impose any cost-sharing requirements (such as a copayment, coinsurance, or a deductible) for immunizations “listed on the Immunization Schedules of the Centers for Disease Control and Prevention).”15
Conclusion
“Working for the pharma companies, there’s just nothing better than getting on the vaccine schedule.” ~Calley Means
The time is ripe for us to have a national conversation about how vaccines are harming our children (and us). Heavy hitters in alt-media, like Carlson, and top politicians, like Trump and Kennedy, have opened the door for us to question openly what exactly these shots do in the long term and what has been happening generationally to our society. The confusion people have had to keep to themselves, or have been shamed and ridiculed for, about watching their children become ill, while doing everything “right,” is coming to an end. We now have decades – very sad and unnecessary decades – of postmarket data to look at. And that data is our children. Our children are sicker than ever before. Adults are more obese with more chronic illness. But while Americans have suffered, pharmaceutical companies have thrived. What’s wrong with this picture?
The more people speak out, the less fear will stop us from seeing the truth. Let’s stand for health freedom by continuing this conversation wherever we can.
Resources
- “Pharmaceutical Industry Summary Page.” Violation Tracker. https://violationtracker.goodjobsfirst.org/industry/pharmaceuticals. ↩︎
- “A National and State Profile of Leading Health Problems and Health Care Quality for US Children: Key Insurance Disparities and Across-state Variations.” Academic Pediatrics 11, no. May-June (2011). https://pubmed.ncbi.nlm.nih.gov/21570014/. ↩︎
- https://www.researchgate.net/figure/The-first-harmonized-vaccine-schedule-1995-For-the-current-childhood-birth-through-18_fig1_267628614 ↩︎
- “Recommended Child and Adolescent Immunization Schedule.” CDC, (2024). https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. ↩︎
- “Historical Perspectives History of CDC.” MMWR Weekly, (1996). https://www.cdc.gov/mmwr/preview/mmwrhtml/00042732.htm#top. ↩︎
- “History and Evolution of the Advisory Committee on Immunization Practices — United States, 1964–2014.” Morbidity and Mortality Weekly Report (MMWR), (2014). https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6342a5.htm. ↩︎
- “CDC Director Overrides Advisory Panel, Recommends Pfizer COVID Boosters for Front-line Workers.” Fierce Healthcare, (2021). https://www.fiercehealthcare.com/hospitals/cdc-director-overrides-advisory-panel-recommends-pfizer-covid-boosters-for-frontline. ↩︎
- “Package Insert: Diphtheria and Tetanus Toxoids Adsorbed USP (For Pediatric Use).” (2005). https://images.procon.org/wp-content/uploads/sites/17/dt_sanofi.pdf. ↩︎
- “Package Insert: INFANRIX.” https://www.fda.gov/media/75157/download. ↩︎
- “Package Insert: INFANRIX.” https://www.fda.gov/media/75157/download. ↩︎
- “Package Insert: DAPTACEL.” https://www.fda.gov/media/74035/download. ↩︎
- “The Introduction of Diphtheria-Tetanus-Pertussis and Oral Polio Vaccine Among Young Infants in an Urban African Community: A Natural Experiment.” EBioMedicine, (2017). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5360569/#:~:text=These%20vaccines%20were%20introduced%20in,with%203%2Dmonthly%20weighing%20sessions. ↩︎
- “The First Rotavirus Vaccine and the Politics of Acceptable Risk.” Milbank Quarterly, (2012). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3460207/#b30. ↩︎
- “The First Rotavirus Vaccine and the Politics of Acceptable Risk.” Milbank Quarterly, (2012). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3460207/#b30. ↩︎
- “Addition of an Addendum to the 2023 Immunization Schedules for Children/Adolescents and Adults.” CDC, (2023). https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-22/09-Mat-Peds-Schillie-508.pdf.